
Understanding COVID-19 Clinical Trials
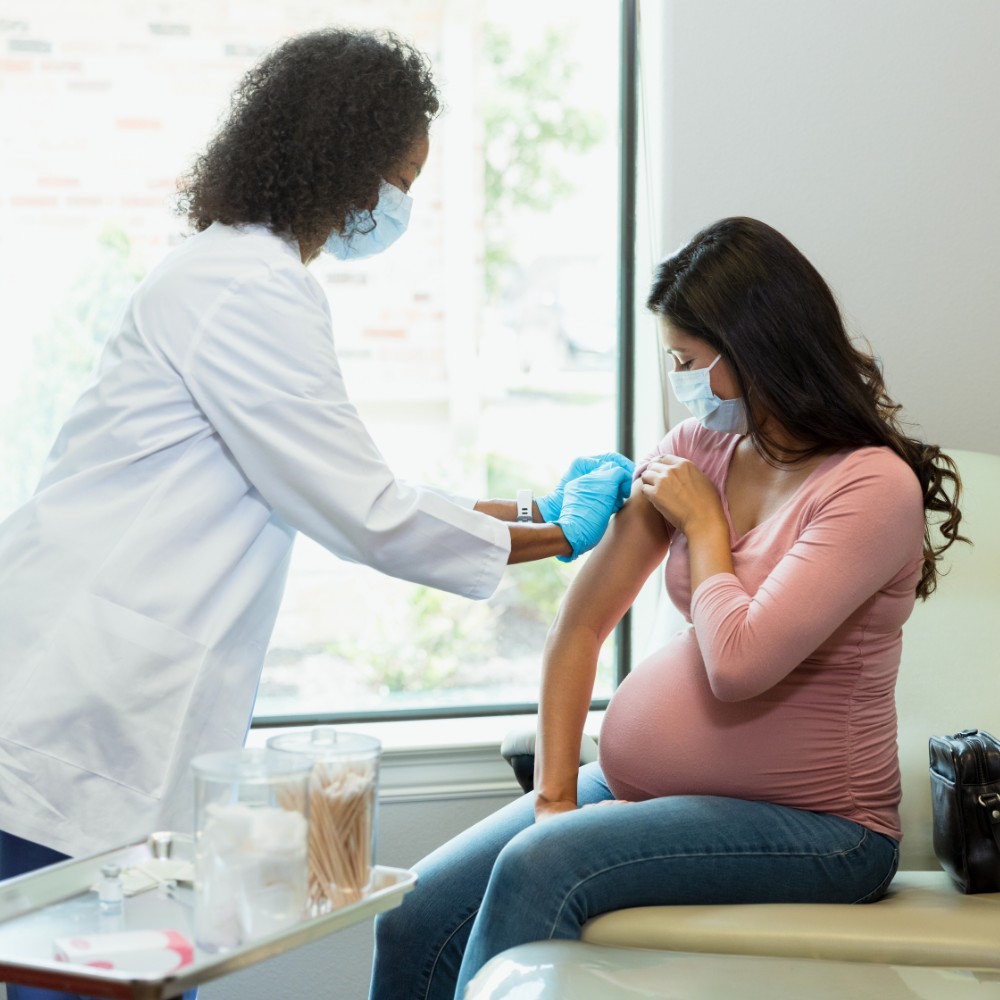
What is a clinical trial?
Clinical trials are medical research studies with volunteers. The purpose of the studies is to determine whether a new treatment or vaccine works and is safe for people to use. After researchers thoroughly test new treatments or vaccines in the lab to make sure they may benefit people, the most promising treatments move into clinical trials.
How clinical trials work and what happens in each phase
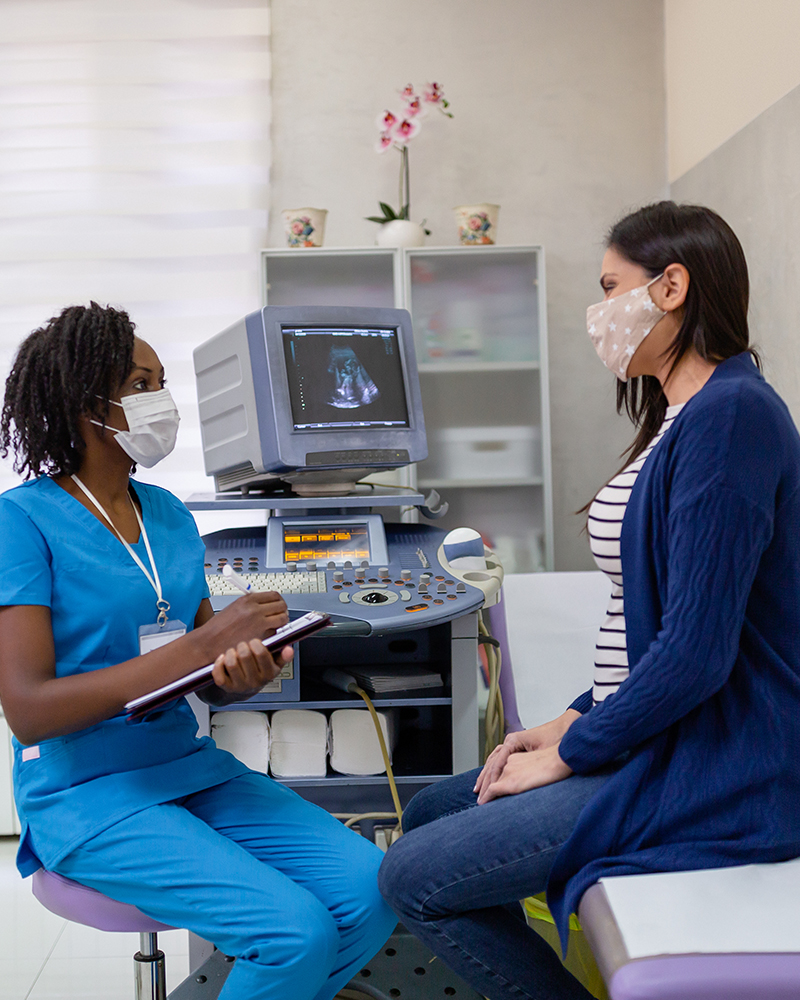
Are there clinical trials to study Long COVID?
NIH is sponsoring research to learn more about Long COVID and to develop ways to prevent or treat these long-term effects. NIH’s Researching COVID to Enhance Recovery (RECOVER) Initiative has launched multiple clinical trials to gather information about the long-term health effects that some people experience after SARS-CoV-2 infection. You can find studies near you by filling out a short online form.
Some RECOVER clinical trials will also test potential treatments for Long COVID. Visit the Long COVID page to learn more about these treatments and how you can participate.
Why should I participate in a clinical trial?
People participate in clinical trials for a variety of reasons. You may want to join a COVID-19 clinical trial if you want to:
-
Make a difference and help end the COVID-19 pandemic
-
Make sure COVID-19 vaccines and treatments will work for as many people as possible
-
Help scientists gain knowledge to fight other diseases and lower the risk of future pandemics
Are clinical trials safe?
If you take part in a clinical trial, your safety and privacy will be protected. Every clinical researcher is required to monitor participants to make sure they’re safe. These safeguards are an essential part of the research.
-
Before they begin, clinical trials must be approved by an institutional review board (IRB). An IRB is made up of doctors, scientists, and people like you and is dedicated to making sure that study participants are not exposed to unnecessary risks.
-
Many clinical trials are closely supervised by a data and safety monitoring board (DSMB). A DSMB is made up of experts in study design, data analysis, and the trial’s targeted condition. These experts, who are not part of the research team, periodically look at the results of the study as it is in progress. If the DSMB finds that an experimental treatment is not working or is harming participants, the board will recommend that the trial be stopped right away.
-
People who join clinical trials must give informed consent. This means that they are told exactly what is going to happen, what the risks are, and what their rights are.
How do I know whether a clinical trial is real?
Clinical trials are very important, but not all of them are legitimate. A real clinical trial will:
-
Never ask you to pay to be in a study
-
Never ask for your Social Security number, bank account, or credit card during recruitment or screening
Most legitimate trials compensate volunteers to help offset the time and inconvenience of participation in a study. The Federal Trade Commission has additional guidance to help you determine whether a clinical trial is real or fake.
Find a COVID-19 Clinical Trial
COVID-19 Clinical Trials
The National Institute of Allergy and Infectious Diseases (NIAID) shares a list of ongoing clinical trials for COVID-19 treatments and vaccines and studies of people who have recovered from SARS-CoV-2 infection.
ClinicalTrials.gov
This searchable database shows federally and privately supported clinical trials studying COVID-19 in the United States and around the world. Use the options under "Filters" to search for trials that match your needs.
Clinical Trial Resources
Including people from every community is important in clinical research.
Learn about the importance of including communities of color in clinical trials.
Vaccines are tested through the different phases of clinical trials.
Participants and researchers describe what taking part in a clinical trial is like.
 An official website of the United States government
An official website of the United States government